INTRODUCTION TO
HYDROGEN POWER
Ph. M. Kanarev
Kuban State Agrarian University
13, Kalinin St., Krasnodar 350044, Russia
E-mail:<[email protected]>
Brief Historical Reference
Theoretical and
experimental results of investigations demonstrate that water plasma electrolysis
can become the most probable source of cheap hydrogen obtained from
water. The author's certificate for the first plasma-electrolytic
reactor was received by a group of scientists from Russia in 1987
[18]. As the reactor was developed at the enterprise of the military industrial
complex, the author's certificate was issued with a signature stamp "For
service use only" and its contents was not published in open press.
Line diagram of the reactor as well as the results of water purification
and disinfecting with the help of plasma being formed in the cathode zone
of the reactor is given in the description of the certificate. No data
on generation of additional energy by plasma and obtaining of hydrogen
are given the description of the author's certificate [18].
The American scientists S. Pons and M. Fleischmann have published
the results of their researches on production of additional energy during
usual electrolysis of water in April 1989. They have announced that cold
nuclear fusion is the source of the energy [2].
Ya.A. Beklyamishev, one of the
co-authors of the first author's certificate for plasma-electrolytic reactor,
has published experimental results which demonstrate availability of additional
energy in plasma-electrolytic process in 1996 with no explanation of the
source of this energy [17]. The novelty of our results in this field
is attested by a patent application with priority date November 15, 1997
[19].
In 1998 the new experimental
data concerning availability of additional energy in plasma-electrolytic
process appeared. A group of scientists from Russia carried out control
tests of one of plasma-electrolytic reactor, it registered officially additional
energy, and this fact was documented with the help of a check test report
dated 22 May, 1998. This report was published in the 22nd issue of
the American magazine "Infinite Energy" [10]. In addition to it, in May
of the same year the third issue of the book "Crisis of theoretical physics"
was published [7] where the data on production of additional energy during
plasma electrolysis of water are stipulated and the source of this
energy is mentioned. In the same year Ohmori and Mizuno, the
Japanese scientists, published their results in transactions of Vancouver
conference on cold nuclear fusion, and in the 20th issue of the American
magazine "Infinite Energy" [3]. Ohmori and Mizuno registered neutron radiation
and emerging of iron, chromium, nickel and carbon on tungsten cathode;
it looked like forcible argument of availability of cold nuclear fusion
when plasma electrolysis of water takes place. It seemed that Ohmori and
Mizuno explained neutron radiation correctly as a result of electrons capture
by protons. But they made a premature conclusion concerning synthesis
of iron, nickel and chromium, because the parts of the devices for distilled
water production contain these chemical elements and organic impurities
contain carbon. Besides, nuclear fusion would give much more additional
energy than they registered.
The first suppositions that during usual and plasma electrolysis
of water the synthesis of atoms of hydrogen, not nuclear fusion, is a source
of additional energy were published in 1996 in the works [14], [16].
Later on this idea was developed and added by new theoretical and experimental
results published in the works [15], [7], [1]. New experimental results
which demonstrate energy expense reduction for obtaining hydrogen during
plasma electrolysis of water have been published in 1999 [1], [19]. Summary
of the main theoretical results taken from the above mentioned sources
and a small part of experimental data are given below.
Introduction
Hydrogen is considered to
be the most prospective energy carrier of future power. Water is its main
source. But all existing ways of hydrogen production from water require
larger power consumption than it is produced when hydrogen is burned. From
the point of view of modern physics and modern chemistry it is a normal
phenomenon, because it corresponds completely to energy conservation law.
But at present the publications concerning
the results of experimental investigations appeared which prove the existence
of such processes when more energy is released than spent for realization
of these processes [1], [2], [3], [4], [9], [10], [17], [19], but neither
modern theoretical physics nor modern theoretical chemistry allow
to explain these results.
The question arose: either energy
conservation law in its modern wording is untenable or the directions on
which modern theoretical physics and theoretical chemistry develop have
exhausted their possibilities. The achievements of modern physics and chemistry
are so significant that statements of such a question seems to be inappropriate,
but the experimenters get new and new results [9] which are at variance
with energy conservation law and make us put this question and search an
answer for it.
The history of the
development of exact sciences shows that if new experimental data which
cannot be explained by exact sciences appear, the sciences return to their
axiomatic basis. Thus, last century when new experimental data on behavior
of light could not be explained, Euclid's axioms were subjected to analysis;
as a result, new axioms and new theories appeared which as it seemed explained
the results of those experiments. Something of the same kind takes place
now. Experimenters have place exact sciences in such a state when it has
become necessary to analyze their axiomatic basis [8], [9]. Such an analysis
has already made a good start [5], [6], [7]. The proofs of the new theoretical
results are rather sufficient to be stated in a scientific article, that's
why we have the only opportunity: to enumerate them and to indicate the
sources in which those who so desire can find details of these proofs.
Theoretical Results
1- It was found out that the theoretical limitations in the development
of exact sciences which appeared last century is an after-effect of absence
of formulation and understanding of the axiom of space - matter - time
unity. In reality space, matter and time are inseparable. Thus, only
these mathematical models reflect reality in which space, matter
and time are presented in undivided state [1], [5], [6], [7];
2 - It follows results from space - matter - time axiom as well as
from kinetic moment conservation law that Planck's constant and energy
of single photons and electrons are vector quantities [1], [5], [6], [7];
3 - Atom and ion spectra formation law controls power behavior of electrons
in atoms and ions [1], [5], [6], [7], [12], [13]:
F = Ei-E1/n2
(1)
where F is energies of emitted and absorbed photons by electrons;
Ei is energies of ionization for electrons; E1
is energies of connections of electrons with the nuclei of atoms and ions
which correspond to the first energy level and are determined from experimental
data according to special methods [5], [6], [7], [12]; n = 2,3,4... is
main quantum number;
4 - Electron has a form of hollow torus and has no orbital movement
in atom, it precesses on atom nucleus like a whipping top; unlike electrical
fields of proton and electron bring them together, and magnetic poles of
the same name limit this convergence [1];
5 - Atoms and ions in molecules connect unlike magnetic poles of electrons
or protons or their unlike electric poles, that's why only three types
of chemical connections exist: electron - electronic, proton - proton and
electronic - proton connections; the law governs formation of energies
Ec of these connections [1], [5], [6], [7], [12], [13], [19]:
Ec = E1/n2
(2)
With due regard for
above mentioned facts photon (energy carrier) has electromagnetic structure
which is shown in Fig. 1, electron - in Fig. 2, atom of hydrogen - in Fig.
3. The structures of molecules of hydrogen are shown in Fig. 5 and
new structures of molecules of water - in Fig. 7...10. In Figs 11...14
one can see formation diagrams of molecules of hydrogen when electrolysis
of water takes place.
Surely, all presented Figs should
have been commented in a proper way, the chemical formula with the help
of which hydrogen production processes are realized should have been given
as well as all energy calculations which accompany the processes shown
in Figs. But limited volume of a scientific article does not allow
to make it. The persons who wish to know the details of the processes being
analyzed can refer to the books in which they are described [1], [5], [6],
[7], [19].
Photons of the whole scale of
electromagnetic radiations, except radio range, have electromagnetic structure
which is shown in Fig. 1. All existing (postulated earlier) mathematical
relations which describe
photon as a particle and as a wave, including Heizenberg's Uncertainty
Principle and Schrodinger equation, are derived from photon model movement
analysis [6].
a)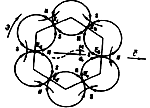 b)
b) 
Fig. 1. Model of photon - energy carrier: a) - theoretical model;
b) simulated model
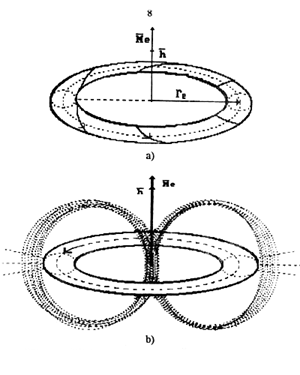
Fig. 2. Diagram of electromagnetic model of electron
In Fig. 2, h is Planck's
constant (angular momentum and electron spin); Me
is magnetic moment of electron; re is
radius of electron theoretical value of which coincides completely with
experimental value of Compton wavelength of electron [1].
In Fig. 2, b)
only a part of electric and magnetic lines of force of electron is shown.
When the whole set of these lines is depicted, electron has a form which
resembles a form of an apple with north and south magnetic poles. All existing
(postulated earlier) mathematical relations which describe electron behavior
are derived from electron model rotation analysis shown in Fig. 2 [1],
[7], [15].
The structure of proton is represented
as a point yet in which the magnetic pole direction with north and south
magnetic poles is clearly seen. It has been found out that the size of
proton is by three orders less than the size of electron and by five orders
less than the size of atom of hydrogen in its excited state. Magnetic moment
of proton is by two orders less than magnetic moment of electron. Magnetic
intensity in geometrical center of proton is by six orders more than in
geometric center of electron [1].
The model of atom of hydrogen
is shown in Fig. 3. Electric forces bring electron together with
proton, and magnetic forces of like magnetic poles limit their
rapprochement. If the directions of magnetic moments of electron and proton
coincide, proton can capture electron, and neutron can be formed. Such
phenomenon takes place during plasma electrolysis of water, and the Japanese
scientists have registered it [3].
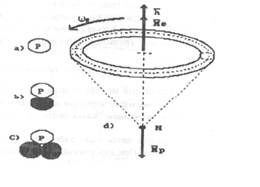
Fig. 3. Diagram of model of atom of hydrogen (Proton -
electronic connection)
The distance between proton and
electron in unexcited atom of hydrogen is near one angstrom (1.058 10-10
m). If ambient temperature is increased, electron is moved off from proton
(nucleus) and passes to higher energy levels (Fig. 4) [1].
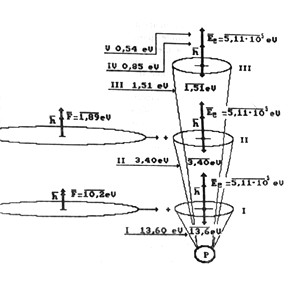
Fig. 4. Diagram of addition of vectors of photon , electron
and energies of bond of electron with atom nucleus of hydrogen 13.6; 3.40;
1.51...eV during absorption: I, II, III - energy levels of electron.
Structures of molecules of
orthohydrogen and parahydrogen are given in Fig. 5. Two atoms of
hydrogen are connected in a molecule in such a way that if electric forces
bring together connecting elements, magnetic forces limit this rapprochement
and vice verse. Analysis of bonds between atoms in molecules of hydrogen
(Fig. 5) shows that this bond is provided by protons and electrons. In
this connection all chemical bonds are divided into electron - electron
bonds, proton - proton bonds and electron - proton bonds. Connection energy
value depends on energy level on which electron is situated at the connection
formation moment. When two atoms are connected in a molecule of hydrogen,
their electrons occupy the levels with bond energies 2.26 eV. Some binding
energies between atoms of hydrogen and oxygen in a molecule of water are
shown in Figs 7...14.
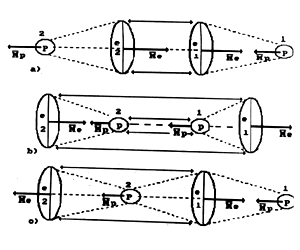
Fig. 5. Diagram of molecule of hydrogen H2: a), b) - orthohydrogen;
c) - parahydrogen
In Fig. 6 the diagram of molecule of
water obtained on the grounds of existing knowledge concerning its structure
is given, but this knowledge is insufficient for explanation of experimental
factors of additional energy obtaining during its electrolysis.
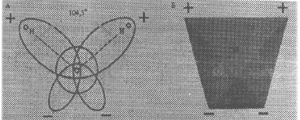
Fig. 6. Old diagram of structure
of molecule of water
It is known that water in
which nuclei of atoms of hydrogen have one (Fig. 3,b) or two (Fig. 3, c)
superfluous neutrons is called heavy water. Now it turns out that molecule
of water can have diverse quantity of electrons. In order to distinguish
molecules according to this phenomenon as well (quantity of electrons),
let us call a molecule which contains complete set (ten) of electrons (Fig.
7) charged molecule and water which contains such molecules only
charged water. Let us call molecule of water which contains minimal quantity
(eight) electrons (Fig. 8) discharged molecule and water which contains
only such molecules discharged water. Let us call molecules of water
which contain nine electrons (Figs 9, 10) semi-charged molecules, and water
containing such molecules semi-charged water. We shall add per cent of
complete charging, discharging and semi-charging. For example, the name
water: 30-60-10 will mean that it contains 30% of charged molecules, 60%
discharged molecules and 10% semi-charged molecules.
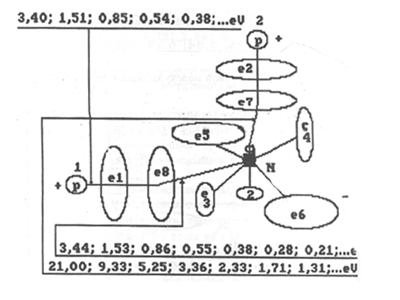
Fig. 7. Diagram of charged molecule of water with binding energies:
1,2,3,4,5,6,7,8 are the numbers of electrons of atom of oxygen; N is
nucleus of atom of oxygen; P is nuclei of atoms of hydrogen (protons);
e1 and e2 are numbers of electrons of hydrogen; e8 and e7 are surface electrons
of atom of oxygen
Water obtained after
burning of hydrogen can serve as an example of charged water. Its molecules
contain ten electrons (Fig. 7). Water obtained in fuel elements after a
transfer of electrons by atoms of hydrogen into electric network and connection
of their protons with electrons of atoms of oxygen can serve as an example
of discharged water (Fig. 8). It follows from these two examples that there
exist molecules of water in which the eighth electron of atom of oxygen
(Fig. 9) or its seventh electron (Fig. 10) are not coupled with electrons
of atoms of hydrogen. These molecules can be called semi-charged, and water
which contains such molecules is called semi-charged water.
Electron of atom of hydrogen at the moment
of formation of a connection with the eighth electron of atom of oxygen
in molecule of water is in the third energy level and binding energy of
1.51 eV. The seventh electron of atom of oxygen is connected with electron
of atom of hydrogen being in the fourth energy level (Figs 7...10).
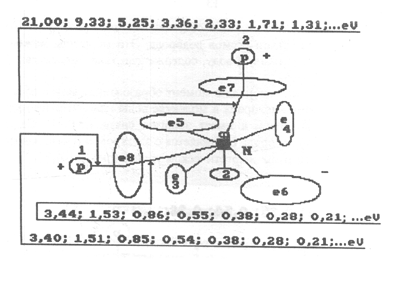
Fig. 8. Diagram of discharged molecule of water with binding energies
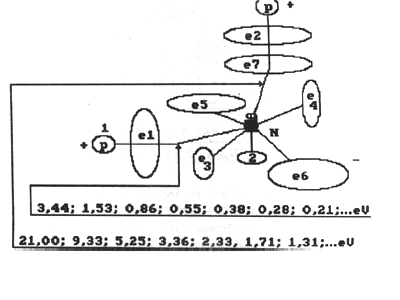
Fig. 9. Diagram of the first model of semi-charged molecule of water
with binding energies

Fig. 10. Diagram of the second model of semi-charged molecule of water
with binding energies
The indicated structures of molecules
of hydrogen and water as well as the diagrams of formation of molecules
of hydrogen during electrolysis of water allow to approach an organization
of the process of obtaining of hydrogen deliberately and due to reduce
the energy expenses for its production [1].
It is known that molecules of water connect with each
other and form clusters. Energy of proton - proton bonds between molecules
of water in the cluster at the temperature of 20°C is equal to
1.49 eV. When water temperature is increased by one degree, this energy
is reduced by 0.0024 eV [1].
If you imagine
a cluster of two molecules of water which have a form of the balls with
a diameter of 100 meters, the protons of atoms of hydrogen in a molecule
of water which are arranged on the surface of these balls and connect them
have millimeter sizes. The least influence on such a system destroys it
creating the conditions for fluidity of water [1].
At first let us pay attention
to the diagram of formation of two atoms of hydrogen from the protons which
have separated from two charged molecules of water and two electrons emitted
by the cathode (Fig. 11). Produced atoms of hydrogen can form a molecule
of parahydrogen (Fig. 11, c) or a molecule of orthohydrogen (Fig. 11, d).
In this case two electrons from electrical network will be spent for the
formation of one molecule of hydrogen, and 5.98 kWh of electric power will
be spent on the formation of one cubic metre of hydrogen [1].
If a molecule of orthohydrogen is formed
according to the diagram given in Fig. 12, an empty cell of the eighth
electron is formed in one molecule of water. It will be immediately occupied
by an electron emitted by the cathode, and an ion of hydroxyl is formed
which will be directed to the anode or will be connected with an ion of
alkaline metal. In this case one electron from the electric network will
be spent for the formation of the molecule of hydrogen, and 2.99 kWh of
electric power will be spent for the formation of one cubic metre of hydrogen
[1].
If two atoms of hydrogen separate from two
charged molecules of water under the influence of electric field and a
molecule of orthohydrogen (Fig. 13) or parahydrogen (Fig. 14) is formed,
the molecules of hydrogen are formed in such cases without participation
of electrons emitted by the cathode, i.e. without expenses of electric
energy.
Thus, a part of molecules of hydrogen
can be formed without the use of electrons emitted by the cathode and a
part - with the use of them. The energy expenses for production of hydrogen
will depend on a relation between these parts.

Fig, 11. Diagram of formation of two atoms and a molecule of hydrogen:
c) parahydrogen or d) orthohydrogen; a), b) charged molecules
of water
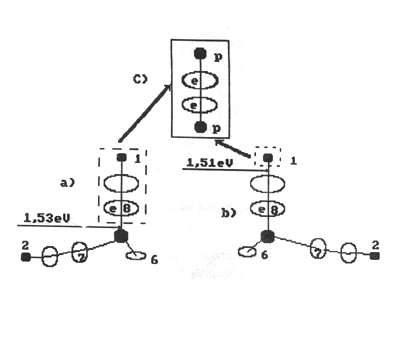
Fig. 12. Diagram of formation of a molecule of orthohydrogen:
a), b) - charged molecules of water; c) - molecule of orthohydrogen
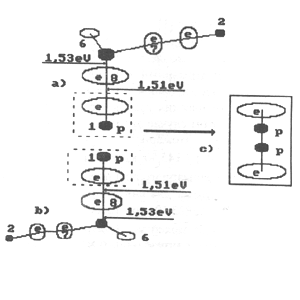
Fig. 13. Diagram of formation of the second model of orthohydrogen:
a) and b) charged molecules of water; c) orthohydrogen
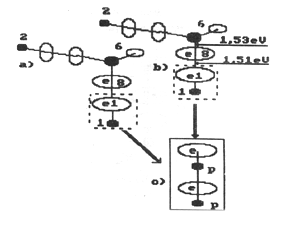
Fig. 14. Diagram of formation of a molecule of parahydrogen:
a) and b) charged molecules of water; c) molecule of parahydrogen
There are several
ways of activation of the described process of formation of molecules of
hydrogen without the use of electrons emitted by the cathode (Figs. 13,
14), i.e. without expenses of electric power. Formation of plasma in the
zone where the processes being described take place, i.e. in the zone of
the cathode, is one of them. A result of one of the experiments connected
with production of hydrogen from water during its plasma electrolysis is
given below [1].
The analysis of a spectrogram of electrolytic
plasma during optimal mode of work of the plasma-electrolytic reactor
shows that it is generated by atomic hydrogen. Before the formation
of molecules of hydrogen the electrons of atoms of hydrogen are detained
in the third energy levels with binding energies with protons of 1.511
eV [1]. In this case the energy released during synthesis of one
mole of atomic hydrogen is (1.511 x 1.602 x 10-19 x 6.02 x1023)
= 145.56 kJ per mole.
109.12 moles of atomic hydrogen are in one liter, that's why (109.12
x 145.56)=15883.50 kJ of energy will be released during the synthesis of
atoms of hydrogen which are necessary for the synthesis of one liter of
water. As there are 54.56 moles of molecular hydrogen in one liter of water
and energy released during the synthesis of one mole of molecular hydrogen
is equal to 436 kJ,
(436 x 54,56) = 23788.16 kJ per l will be released during the synthesis
of one liter of water.
285.80 kJ of energy are released during burning of hydrogen and synthesis
of one mole of water. 55.56 moles of molecules of water are in one liter
of water. Thus, (285.80 x 55.56)=15879.09 kJ of energy will be released
during synthesis of one liter of water by means of burning of hydrogen
[1].
If we sum up the energy which
is released sequentially during the synthesis of atomic and molecular
hydrogen and molecules of water reckoning per l we shall get:15883.50+23788.16+15879.09=55550.75
kJ.
Index of general power efficiency of plasma-electrolytic
reactor No. 3
In order to determine of the general
index of efficiency of a plasma-electrolytic reactor it is necessary
to know power expenses for the destruction of molecules of one liter of
water when its plasma electrolysis takes place. Let us suppose that such
power expenses are unknown to us and let us take power expenses for decomposition
of one liter of water to hydrogen and oxygen as a basis using the best
modern electrolytic methods [11].
There are 1222.2 liters
of hydrogen in one liter of water [1], and the best modern electrolysers
require nearly 4 kWh for the production of 1000 litres of this gas [11].
Therefore, (1222.2 x 4)=4.9 kWh or 4.9 x 3600=17640 kJ are used for the
production of hydrogen from one litre of water. This magnitude of power
can be taken as a basis when calculating the general index of efficiency
of the plasma-electrolytic process. In this case the general index of efficiency
of the plasma-electrolytic reactor will be as follows: K = 55550.75/17640=3.15
[1]. And what does an experiment show?
First of all, the experiment demonstrates
that the plasma-electrolytic reactor generates power as heat of a
heated solution, vapor of a heated solution, steam of various temperatures,
hydrogen and oxygen as well as light radiation, noise and high frequency
electrical oscillations.
In this experiment the reactor No. 3 was adjusted
to the operation mode with minimal steam discharge and maximal gas
discharge and is occupied with a heat exchanger for steam condensation.
Gas output rate after condensation was measures with the help of an anemometer.
The expense of cooling liquid (water) and the change of its temperature
as well as time and indications of the devices which measures the expense
of electric power were registered [1], [19].
Results of experiment
Indices
|
1 |
2 |
3 |
Average |
| 1 - duration of experiment ,dt s |
300 |
300 |
300 |
300 |
| 2 - expense of cooling water m, g |
8600 |
9250 |
8750 |
8867 |
| 3 - water temperature at the input into the cooler t1,
degrees |
24 |
24 |
24 |
24 |
| 4 - water temperature at the out- put from the cooler t2,
degrees |
29.0 |
28.5 |
29.5 |
29.0 |
5 - difference of water temperature
t= t2-t1
degrees |
5.0 |
4.5 |
5.5 |
5.0 |
| 6 - output of gases (hydrogen) according to the indications of anemometer
W, liter |
19.2 |
20.7 |
25.5 |
21.8 |
| 7 - electric power counter disk revolutions during the experiment n,
revolutions |
23.5 |
24.0 |
29.0 |
25.5 |
8 - electric power consumption, kJ
E1=n3600/600 |
141.0 |
144.0 |
174.0 |
153.0 |
| 9 - voltmeter readings V |
220.0 |
220.0 |
220.0 |
220.0 |
| 10 - ammeter readings I, A |
1.66 |
1.75 |
1.89 |
1.77 |
11 - electric power consumption according to the indications of voltmeter
and ammeter, kJ
E2=IVdt |
110.0 |
115.5 |
124.7 |
116.7 |
12 - power used to heat cooling water, kJ
E3=Cmdt; C=4.18 |
179.7 |
174.0 |
201.2 |
185.0 |
13 - power content of produced hydrogen E=W0.09x142
where 0.09 is mass of 1 litre of H2, 142
is power content of 1 g of H2 hydrogen, kJ |
245.7 |
264.1 |
326.6 |
278.8 |
14 - sum of energies generated by the reactor, kJ
E0=E3=E4 |
425.4 |
438.1 |
527.8 |
463.8 |
| 15 - COP of the reactor according to counter readings
K1=E0/E1 |
3.1 |
3.2 |
3.0 |
3.1 |
16 - COP of the reactor according to readings of voltmeter and ammeter
K2=E0/E2 |
3.8 |
4.0 |
4.2 |
4.0 |
| 17 - electric power consumption for production of one cubic metre of
hydrogen, kWh per mexp3 |
2.0 |
1.9 |
1.9 |
1.9 |
|
|
|
|
|
Note: hydrogen produced after steam condensation
can contain impurities of other gases: oxygen and ozone and possibly helium,
but we did not manage to carry out such analysis, that's why energy
value E4 should be clarified.
The following matters have
not been taken into consideration: energy of oxygen which has appeared
on the anode cavity of the reactor; external power losses (the heat exchanger
had no thermal insulation) as well as luminous radiation power. It is possible
to neglect other types of energy which have not been taken into account
(noise, high frequency electric oscillations).
CONCLUSION
General index of plasma-electrolytic
process efficiency obtained during experiments was within the values predicted
by the theory. It is important that energy consumption for production of
cubic metre of hydrogen is half reduced. As burned hydrogen energy and
energy used for its production is equal at nearly 3.5 kWh per cubic metre
of this gas, hydrogen becomes competitive energy carrier when consumption
is nearly 2 kWh per cubic metre. Until recently there was no theory which
could predict the possibility of electric power consumption reduction for
hydrogen production. There were no experimental data confirming such theoretical
forecasts [2], [3], [4].
Theoretical
results are available now which allow to forecast such behavior of molecules
of water when energy consumption for hydrogen production is less than energy
generated when burning produced hydrogen [1]. The experiments being conducted
show that there is a possibility to include hydrogen produced from water
into competitive energy carriers [1], [19].
REFERENCES
[1] - Kanarev Ph. M. Water as a New Source of Energy. Krasnodar 1999.
150 pages. (In Russian)
[2] - Harold l. Fox. «Nuclear Cold Fusion: essence, problems,
its impact on the world. Opinion from USA». Production group SVITEX.
M.: 1993, 183 pages (in Russian).
[3] - Ohmori T., Mizuno. "Excess Energy Evolution, New Element Production,
and Electromagnetic Wave and/or Neutron Emission of Light Water Electrolysis
with a Tungsten Cathode" Infinite Energy, vol. 4, Issue 22, 1998, p.p.
14-17.
[4] - Paramahamsa Tewari. Violation of Law of Conservation of Charge
in Space Power Generation Phenomenon. The Journal of Borderland Research,
USA - Vol. XLV, No. 5. September - October, 1989.
[5] - Kanarev Ph. M. On the Way to the Physics of the XXI Century.
Krasnodar. 1995. 269 pages. (In English).
[6] - Kanarev Ph. M. Analysis of Fundamental Problems of Modern Physics.
Krasnodar. 1993. 255 pages. (In Russian)
[7] Kanarev Ph. M. Crisis of Theoretical Physics. The third edition.
Krasnodar 1998. 200 pages (In Russian)
[8] - Santilli R. M. Physical Laws of the Emerging New Energies as
Predicted by Hadronic Mechanics. I: Insufficiencies of Quantum Mechanics.
Infinite Energy. 1998, V 4, Issue 22, p.p. 33...49.
[9] - ICCF - 7 ACCEPTED ABSTRACTS. Infinite Energy. V 4, Issue 20,
p.p. 59...69.
[10] - Kanarev Ph. M. Protocol of Control Experiments for the Plasma-electrolysis
Reactor N3.Infinite Energy. V.4, Issue 22, 1998. P.p. 31...32.
[11] - Bilain et avenir "systeme" hydrogene. Pt. I. Production transport
et stockade/Logette S. Leclere J.-P., Goff P.Le, Billermaux J.//Entropic.-1955.-31,
No. 188-189. P.p. 95-99.
[12] - Kanarev Ph. M. Analytical Theory of Spectroscopy. Krasnodar
1993. 88 pages. (In English).
[13] - Kanarev Ph. M. Law of Formation of Spectra of Atoms and Ions.
Collection of scientific articles of international conference "Problems
of Space, Time, Gravitation". Part II. Polyengineering. St.-Petersburg
1997, p.p. 30-37. (In Russian).
[14] - Kanarev Ph. M. Crisis of Theoretical Physics. The first edition.
Krasnodar 1996. 143 pages. (In Russian and in English).
[15] - Kanarev Ph. M. Crisis of Theoretical Physics. The second edition.
Krasnodar 1997. 170 pages. (In Russian).
[16] - Kanarev Ph. M. The Secret of "the Cold Fusion". Proceedings
of the International Scientific Conference of New Ideas in Natural Sciences.
Part I. "Problems of Modern Physics", St.-Petersburg, June 17-22, 1996,
p.p. 305-310.(In English).
[17] - Beklyamishev Yu. A., Beklyamisheva G. Yu. A New Direction in
Energetic. Proceedings of the International Scientific Conference of New
Ideas in Natural Sciences. Part I. "Problems of Modern Physics", St.-Petersburg,
June 17-22, 1996, p.p. 311-313. (In English).
[18] - Zykov E.D., Babenchik F.V., Beklyamishev Yu. A., Likhonosov
S.D., Semushkin V.V., Polushin A.A. Method of purification and disinfecting
of solutions and device for its realization. Author's certificate SU 1624924
A1. Application No. 4257400/26 dated June 3, 1987. Invention description
6 pages. USIIPE of the State committee on inventions and discoveries by
GCNT of the USSR.
[19] - Kanarev Ph.M. The Source of Excess Energy from Water. Infinite
Energy. V5, Issue 25. 52-58 pag.
Back to main page
 b)
b) 










